The study in a few words
SHAPERS is a pragmatic study meaning a study designed to help choose between standard treatment options, involving several centres in Belgium, which will include around 230 patients to compare two treatments that are considered standard.
In total, the study participation will last around 6 years, with approximately 5 years of post-treatment follow-up.
The main procedures that will be carried out are identical to those that form part of the usual treatment (without participation in the study), i.e medical consultations, blood tests, magnetic resonance imaging (MRI) of the rectum and thoraco-abdomino-pelvic CT scan or thoracic CT scan and abdomino-pelvic MRI.
On the other hand, patients will have to complete 4 quality of life questionnaires which are specific to the study and take around 10 to 15 minutes to complete, for a total of 3 times during the study and 13 times during follow-up.
Objective:
The aim of the study is to demonstrate that a treatment with radio(chemo)therapy alone before surgery could be as effective as a « triple treatment » (radio(chemo)therapy, and chemotherapy before surgery), while reducing adverse effects in elderly patients.
Study Design
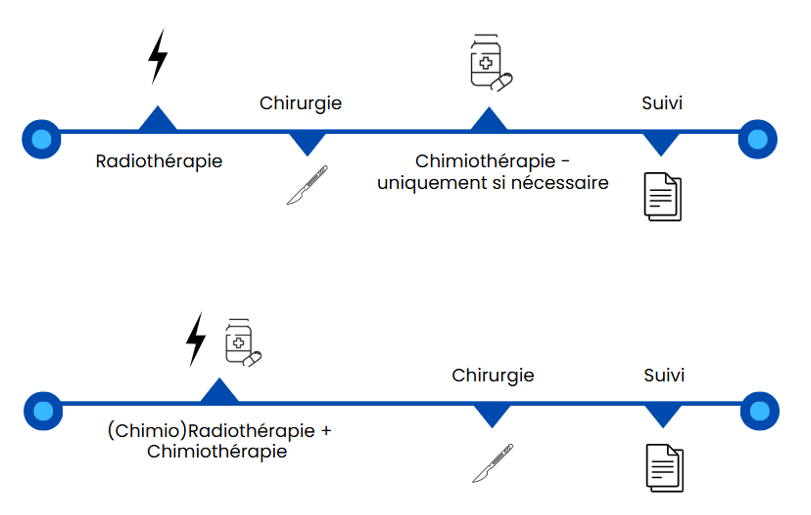
Procedures and conduct of the study:
Participants will be included in the study for approximately 6 years. This period includes the selection, treatment and follow-up phases:
Selection phase (approximately 28 days):
Once the informed consent form has been signed, a number of tests will be carried out to check that the patient meets all the conditions required to take part in the study. During the selection phase, the following tests will be carried out: blood test, MRI of the rectum, thoraco-abdomino-pelvic CT scan or thorax CT scan and abdomino-pelvic MRI. These tests are identical to those performed as part of routine management (with participation in the study). In addition to these standard tests, patients will be asked to complete 4 quality of life questionnaires, which take around 10 minutes to complete. If the patient meets all the inclusion conditions, he or she will enter the study and begin the treatment phase.
Treatment phase:
Depending on which arm of the study the patient is randomised to, the patient will receive one of the treatments explained below:
- Conventional neoadjuvant Therapy (CNT): this consists of radiotherapy or radio(chemo)therapy followed by the surgery. Only if necessary, will the patient will receive chemotherapy after surgery.
- Total neoadjuvant Therapy (TNT): this consist of a treatment that combines chemotherapy and radio(chemo)therapy before the surgery.
For patients who show a complete clinical response, i.e. if several criteria indicate that the tumour has completely disappeared, a « watch and wait » approach is authorised instead of surgery. If the patient is ultimately not operated on, close follow-up will be required (which is normal clinical practice).
Follow-up phase (5 years):
Once the treatment has been completed and the end-of-treatment visit is over, the patient will enter the 5-year follow-up phase. During the follow-up phase, the frequency of visits and the frequency and type of tests that patients will undergo are in line with standard clinical practice (they will undergo the same visits and tests even if they decide not to take part in the study), with the sole exception of the quality- of- life questionnaires.
Extended follow-up phase:
From the 6th year onwards, the investigators will check the patient’s vital status annually using the patient’s national register number.
